Client Overview
Europe based leading technology manufacturer operating in the optics and optoelectronics industries.
Business Need
The client was looking for a testing partner with expertise in healthcare domain to help them get FDA approval for their class-C, microscopic medical device for microsurgeries to be launched into the market
VOLANSYS Contribution
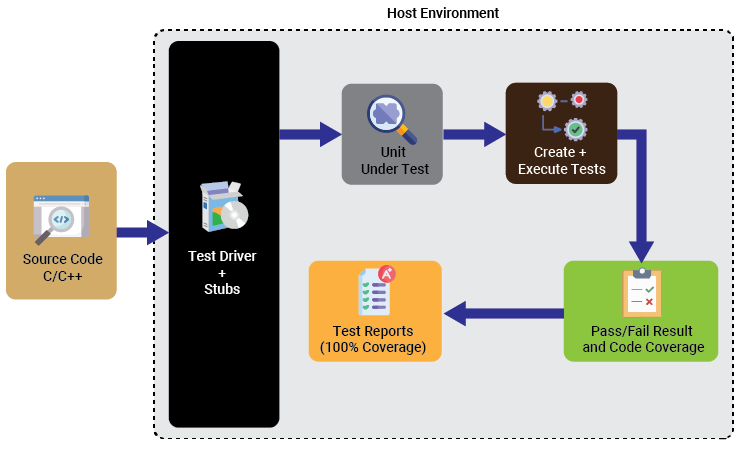
- Developed unit test cases & performed white box testing
- Environment set-up in VectorCAST using tool chain
- Design verification for each function
- Detected logic errors & identified defects introduced by changes in code
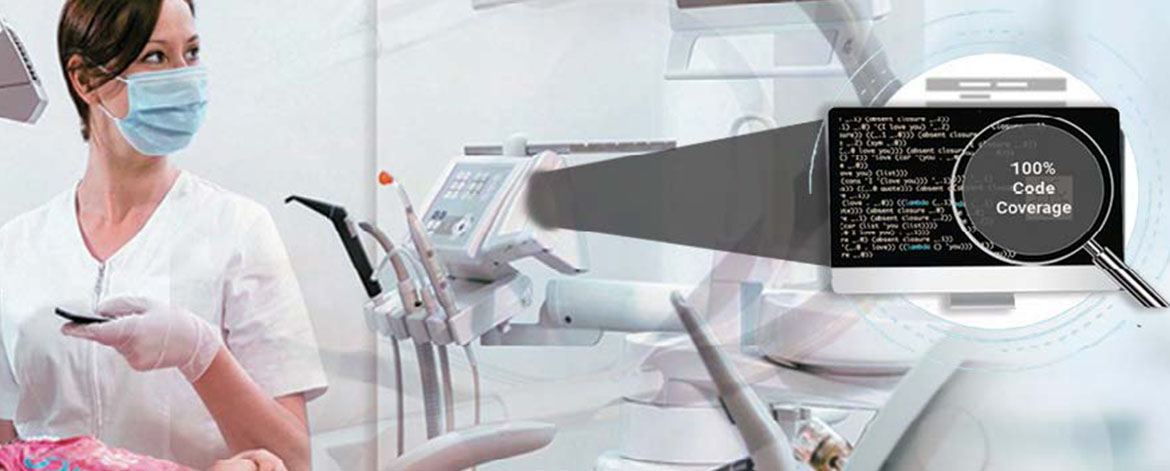
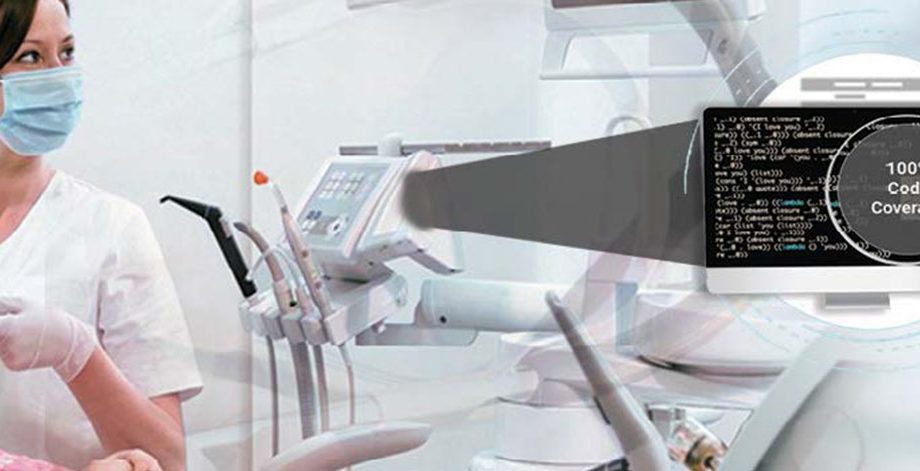
- Code coverage analysis/ management/ verification
- Created compound test cases for control flow verification
- Added user code to define custom function Input/ Output
- Created a regression script for repeatable use (.bat, .env, .tst. files format)
- Performed unit testing with different code coverage type: statement, branch, MC/DC
- Test case report generation (full report, matrix report, test case management report, aggregate coverage report)
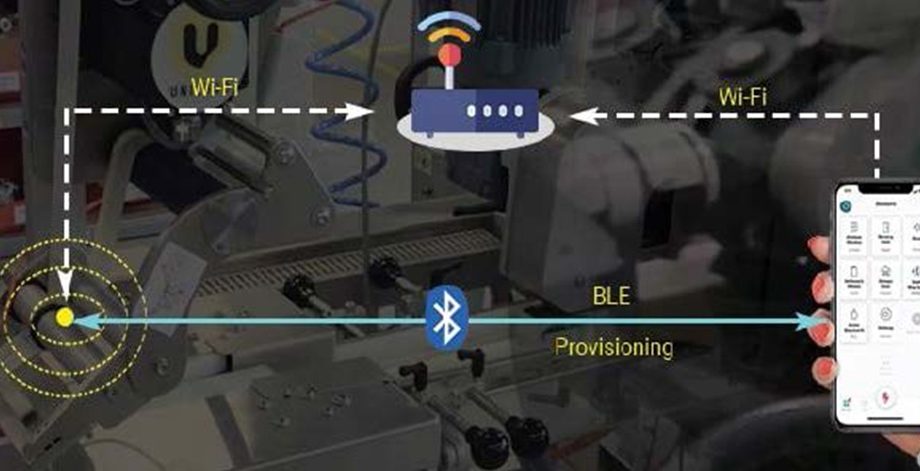
Solution Diagram
Benefits Delivered
- Reduced compliance failure risk by testing the source code (C/C++) via FDA approved tool – VectorCAST
- Improved medical device code accuracy to 90% plus with efficient testing approach
EXT:- 287
Success Stories
Medical Device Testing
Europe based leading technology manufacturer operating in the optics and optoelectronics industries. The client was looking for a testing partner with expertise in healthcare domain to help them get F
Automated Monitoring Of Labelling Solution For Fresh Farm Produces
Leading UK based Industrial labeling system provider of the farm products. The client wanted to develop stable and scalable Wi-Fi mesh solution of 50 nodes to manage and monitor labelling solution for
Extended Support Team For Platform Development
US based leading semiconductor manufacturer offering microcontrollers and processors to sensors, analog ICs and connectivity. Having a large customer base, the client was looking for a technical suppo
End-To-End Testing For Automotive Wheel Management System
US based technology company serving the transportation industry. The client was looking for a testing partner to help them test their end-to-end wheel management system with field trials that would en
Dosage Infusion Pump Firmware Development & Quality Engineering
A US based leading pharmaceutical company. The client wanted to develop a FDA compliance medical Infusion pump device to automate and control desired medical dosage flow rate, ensure accuracy in the a